I don’t read the New York Times regularly, and it’s not because I don’t like them specifically — I just don’t read newspapers anymore. I get my news from a bunch of different sources depending on what I want to know. I used to try listening to NPR, but I hate politics and I’d rather listen to music on the way to work. I also used to read MSNBC online until I finally became violently allergic to them. Nonetheless, I recently came across an NYT editorial cruising around on Facebook that sorta got my attention, because it’s about toxic chemicals. It’s by a columnist named Nicholas Kristof and you can read it at this link. Here’s the important parts:
–
“Just consider formaldehyde, which is found in everything from nail polish to kitchen countertops, fabric softeners to carpets. Largely because of its use in building materials, we breathe formaldehyde fumes when we’re inside our homes.
Just one other fact you should know: According to government scientists, itcauses cancer. The chemical industry is working frantically to suppress that scientific consensus…The chemical industry is represented in Washington by the American Chemistry Council, which is the lobbying front for chemical giants like Exxon Mobil, Dow, BASF and DuPont. Those companies should understand that they risk their reputations when they toy with human lives.
The American Chemistry Council first got its pals in Congress to order a $1 million follow-up study on formaldehyde and styrene. Then it demanded, through a provision drafted by Representative Denny Rehberg, a Montana Republican, that no money be spent on another Report on Carcinogens until the follow-up was completed — meaning a four-year delay until the next report. Stay tuned for an industry effort to slip some such provision into the next budget legislation.”
–
OK, so I feel conflicted. On the one hand, it does sound like the American Chemistry Council is probably being obstructionist, and I can’t say I’m surprised. Out here in CA, environmentalists like yours truly have been trying to get plastic bag bans passed for years, and the ACC is always fighting us. I saw this shiny ACC website about plastics one time which assured readers that plastics are an environmentally responsible choice and that demand for recycled plastic is strong, which isn’t true outside of certain niche applications. (Hell, I can remember working at a supermarket as an undergrad, and every night as directed by management we’d take the plastic bags from our plastic bag recycling bin and chuck them in the dumpster.) So no, I’m not a big fan of the ACC’s stance on many things.
On the other hand, I’m not crazy about the way Kristof is characterizing formaldehyde, because he contains formaldehyde also, whether he knows it or not. You naturally have formaldehyde in your bloodstream at relatively low levels. Certainly formaldehyde is a dangerous chemical, but the question is how much is a dangerous dose and what kind of dose are we getting from our building materials and so forth? because whether he knows it or not, Nicholas Kristof is emitting tiny amounts of formaldehyde too.
And it’s a shame Kristof doesn’t go into all this, because there IS a controversy here, even if he’s portraying it in an simplistic good-vs.-evil, gnomes-vs.-orcs, alien-vs.-predator kind of way. The controversy seems to be urea formaldehyde. I believe they use this in particle board and also in some insulation. It’s a polymer you make by reacting (surprise) urea and formaldehyde. This stuff does leak a little formaldehyde, and yeah, I can definitely see why you’d want to know exactly how much it’s leaking (because again, how much is an important thing to know). Moreover, I think there was a recent controversy about some hair smoothing products that apparently contained unsafe levels of formaldehyde.
So these are legitimate controversies. But the way he’s explaining the problem isn’t logical.
This Washington Post article is what Nick Kristof’s article SHOULD have looked like IMHO:
–
“particleboard isn’t part of the green-building pantheon. That’s because of the resin glue that binds the wood fibers and provides structural strength. It contains formaldehyde, which emits gases into the air from the finished boards. At surprisingly low levels, these formaldehyde emissions produce a pungent odor, and they can pose a health risk.
For decades, formaldehyde emissions have been known to cause eye, nose and respiratory irritations in sensitive people. More recently, the World Health Organization classified formaldehyde, which is used in many other building and consumer products, as a carcinogen.
However, unlike dioxin, a man-made chemical that is harmful in any amount, formaldehyde can be found in every living organism, including humans. Every breath we exhale contains a trace amount.
Wood is another natural emitter of formaldehyde. A room with solid wood furniture will have trace amounts of formaldehyde in the air.
Clearly, formaldehyde emission in these minute amounts is safe. The question vexing experts for more than 20 years is how far you can go beyond “minute.” Particleboard inevitably becomes part of such discussions because it is one of the primary sources of indoor formaldehyde emissions.
How much formaldehyde are we talking about? Though the amounts are very small, we are exquisitely sensitive beings, said Marilyn Black, an environmental chemist and founder of the Greenguard Environmental Institute, a nonprofit group that tests and certifies formaldehyde emission levels in more than two dozen building-product and furnishing categories.
Some people may be affected by emission levels as low as 0.03 parts per million, but most become aware of the odor and experience nose and throat irritation at 0.1 to 0.2 parts per million. Above 0.3 parts per million, almost everyone will notice their eyes watering and nose and throat becoming irritated, Black said.
On average, formaldehyde emission from particleboard is about 0.2 parts per million.
So what level of emissions is considered healthy? For the general population, many health experts have advocated an exposure rate below 0.1 parts per million. Greenguard is more conservative. Products that it certifies emit formaldehyde at levels below 0.05 parts per million, the standard for the state of Washington and the background level typically found in homes and offices, Black said.”
Now this article isn’t perfect either, but it’s a huge improvement on Kristof’s because it actually talks about dose and exposure and some of the issues I raise so often in connection with chemicals on this blog. So if Kristof’s article had read like that, I would have been truly impressed. I would have been pleasantly dumbfounded if he’d included the chemical structure of formaldehyde. And if he’d compared formaldehyde to acetone and phosgene and talked about trends in the periodic table, he would have blown my mind.
I guess he didn’t do that though. So I’ll do it here.
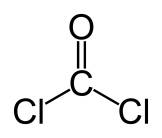
Take a look at the three structures below. The one on the far left is phosgene, the one on the far right is acetone, and the one in the middle is formaldehyde. All three are colorless (as you’d expect — no large networks of alternating double-single bonds or metal complexes here). The thing I want to point out is the trend in toxicity. Acetone isn’t very toxic; formaldehyde is waaay more dangerous and a carcinogen to boot. And phosgene is so out-of-this-world toxic it makes formaldehyde look tame by comparison. That’s why they used phosgene as a poison gas in World War I. It’s nasty nasty stuff — lethal at ridiculously low levels, and it’s a slow and painful death.
So here’s a riddle for you. You can see the trend, but why is there a trend? Why is phosgene more toxic than formaldehyde, and why is formaldehyde more toxic than acetone? What’s going on?
(Hint: notice there’s only one difference between the three compounds: what kind of groups are attached to that central carbon. So what’s different about chlorine, hydrogen and a methyl group (CH3)? And no, it’s not just about size. Yeah, size matters, but it’s less important than you think, you know.)



is it more toxic because of changes to polarity and solubility?
Solubility not really, not in this case anyhow.
But you’re absolutely right when you say that polarity is the key here. More on this below.
I suspect the differences in toxicity could be due to the electron withdrawing ability of the atoms bound to the central carbon atom. Which in turn leads to the central carbon becoming somehwat electron deficient more likely to react with nucleophilic atoms in biochemical systems.
That might be a little off. Third year organic was a couple of years ago and I mostly do physical/inorganic chem with minerals these days.
Bingo! bonus brownie points. To explain in more detail, here’s what’s going on (and I’ll try and keep this at a pretty simple level).
As you go towards the upper right hand corner of the periodic table, elements get more selfish about how they share electrons. Fluorine is the worst electron-hog on the periodic table, with oxygen taking second place.
In all three of these compounds, you have an oxygen double-bonded to a central carbon, meaning they are sharing four electrons — and not sharing those very equally, either, since oxygen is much more of an electron-hog than carbon. So the carbon ends up a little electron-poor. In acetone, that’s not too much of a problem, because you’ve got two CH3 or methyl groups attached, and the central carbon can suck a little electron density away from the methyls to make up for its own deficiencies. So the central carbon isn’t too badly off.
In formaldehyde, however, all you have is those two wimpy hydrogens, and they’re not much of a help — certainly not so much as the methyl groups. So the central carbon in formaldehyde is very electron-poor. Things that are very electron-poor want to react with things that are electron rich. DNA and proteins in your cells contain electron-rich nitrogens that can react with something like formaldehyde.
Now PHOSGENE, by contrast, has two chlorines attached to the central carbon. Chlorine, like oxygen, is an electron-hog, so now you’ve got these chlorines actively sucking electron density AWAY from the central carbon. So phosgene is just about desperate to react with anything that can share a little love (or electron density, I guess). And so it will react with DNA and proteins. To make matters worse, chlorine is a pretty decent “leaving group”, meaning it’s not too terribly difficult to kick it out of the molecule in the form of a chloride ion. So phosgene can also react with water to yield carbon dioxide and hydrochloric acid. In your lungs, then, it’s 1) reacting with water to yield hydrochloric acid, which is a problem for obvious reasons and 2) reacting with proteins. In other words, it wreaks havoc and is thus far more toxic than either formaldehyde or acetone.
I really appreciated your posting. I’m no chemist, having only a basic understanding from high school classes, but I was largely able to follow your presentation and explanation. Thanks for the great article!
Reblogged this on chemistry is the answer !.
Pingback: Links 10/11/12 | Mike the Mad Biologist
“On average, formaldehyde emission from particleboard is about 0.2 parts per million.”
What on earth does that mean?
If you put a chunk of particle board into a closed container with air the equilibrium concentration of formaldehyde in the air will be 0.2ppm?
A tonne of particle board will emit a total of 0.2 grams of formaldehyde?
It will emit 0.2ppm per stone per fortnight?
If you have three particle board bookcases and a sheet on hand with the rest of your lumber your house will have 200ppb of formaldehyde in the air?
Arithmetic. It matters.
Oh, you’re absolutely right. her article is unclear. but ask yourself this — which is better, her article or kristof’s? no contest there. her article at least gives readers some idea how particle board compares to ambient air. kristof’s article makes it sound as though formaldehyde didn’t exist until we started making particle board or something. so yea, i agree, her article could use some work. but you know, i hold the media to a lower standard. as long as they’re halfway right, i’m happy.
Also, keep in mind that when phosgene reacts with a nucleophile, say a terminal amine (pun intended) inside your lungs,it kicks out HCl right where you need it the least.
Same goes for nitrogen and sulfur mustards. Scary shit.
The amazing thing about that is that nitrogen mustards were once used as chemotherapy agents (back in the 50s, I think, although I don’t know all the history on that.)
To the best of my knowledge, they still are!
DOH! you’re absolutely right — not sure what I was thinking. I guess I was thinking of just mustine specifically, which is the nitrogen analog of mustard gas, and I don’t think anybody uses mustine anymore. But cyclophosphamide is a nitrogen mustard, and that’s in very common use.
Pingback: I’ve got your missing links right here (13 October 2012) » Gocnhin Archive
Howdy terrific website! Does running a blog similar to this take a great
deal of work? I’ve virtually no expertise in computer programming however I had been hoping to start my own blog in the near future.
Anyhow, should you have any recommendations or tips for
new blog owners please share. I know this is off subject however
I simply had to ask. Cheers!